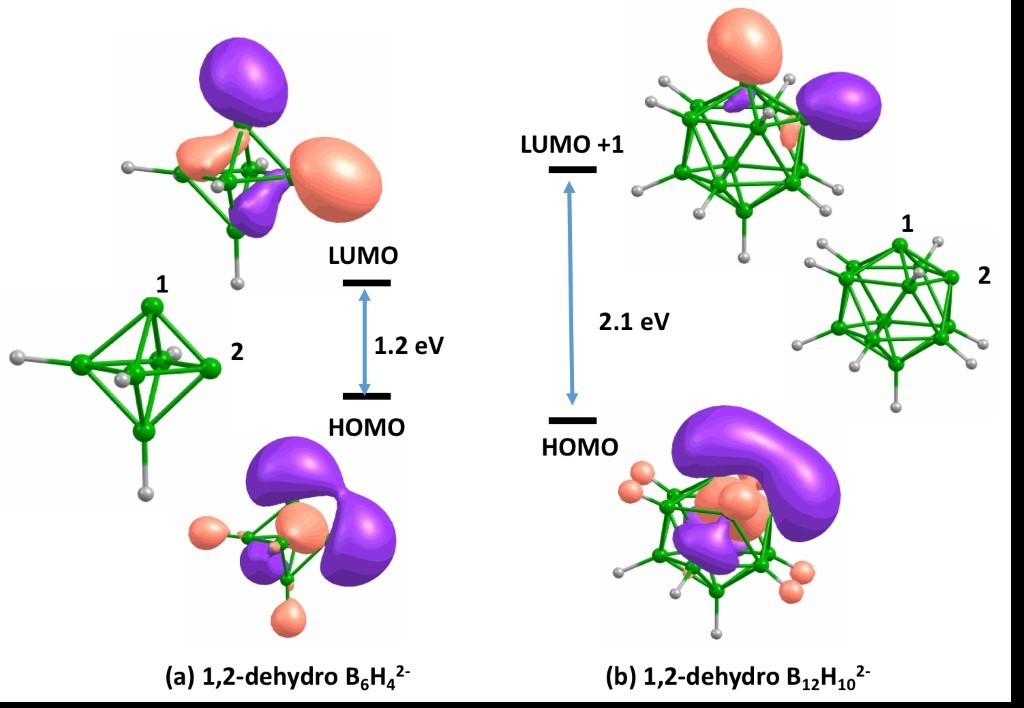
The stability of di-dehydrogeno polyhedral boranes is determined by the overlap of exohedral orbitals, which, in turn, depends on the size of polyhedral.
At Prof. E.D. Jemmis’ Lab at Department of Inorganic and Physical Chemistry (IPC), current emphasis of Research is on several areas: (a) Chemistry of elemental boron, boron clusters, boranes, metallaboranes and metal borides, (b) Transition metal organometallics, activation of small molecules, C-H bond activation, agostic interactions, C-C coupling reactions. H-bond, and weak interactions across the periodic table (d) Chemistry of heavier main group elements
“We study the structure and reactivity problems of real-life molecules, clusters and solids using theoretical techniques ranging from the simplest of molecular orbital methods to the sophisticated electronic structure theory depending on the system at hand and the questions that are to be answered. Special emphasis is placed in weaving threads between problems in one area to another; between polymorphs of elements and their compounds, between organic and organometallic chemistry, amongst the chemistry of various main group elements; Bonding, Structure and Reactions across the Periodic Table of Elements. We place great importance in not only getting numbers as an answer to a problem, but also in finding out why the numbers turn out the way they do, based on overlap of orbitals, perturbation theory, and symmetry, and in devising transferable models”, says Prof. Jemmis. The lab has utilized about 1.85 million core hours of SahasraT in 2019. Members of the lab that use SERC facilities spans across academic and research programmes of IISc, ranging from IISc-UG programme, summer research programme upto postdoctoral researchers. One PhD student, Dr. Naiwrit Karmodak, who completed PhD during 2019 had his thesis adjudged to be the best thesis of the Dept for the year.
Publications and outcomes
1. Designing M-bond (X-M··· Y, M = transition metal): σ-hole and radial density distribution. J. Joy, E.D. Jemmis. J. Chem. Sci. 131 (2019) 1-8.
2. Organoaluminum cations for carbonyl activation. R. Kannan, R. Chambenahalli, S. Kumar, A. Krishna, A.P. Andrews, E.D. Jemmis, A. Venugopal. Chem. Commun. 55 (2019) 14629-14632.
3. Stabilization of Classical [B2H5]-: Structure and Bonding of [(Cp*Ta)2(B2H5)(μ-H)L2] (Cp*=η5-C5Me5; L=SCH2S). K. Saha, S. Ghorai, S. Kar, S. Saha, R. Halder, B. Raghavendra, E. D. Jemmis, S. Ghosh. Angew. Chem. Int. Ed. 58 (2019) 17684-17689.
4. Isolation of base stabilized fluoroborylene and its radical cation. S.K. Sarkar, M.M. Siddiqui, S. Kundu, M. Ghosh, J. Kretsch, P. Stollberg, R. Herbst-Irmer, D. Stalke, A.C. Stückl, B. Schwederski, W. Kaim, S. Ghorai, E.D. Jemmis, H.W. Roesky. Dalt. Trans. 48 (2019) 8551-8555.
5. A Dicationic Bismuth(III) Lewis Acid: Catalytic Hydrosilylation of Olefins. S. Balasubramaniam, S. Kumar, A.P. Andrews, B. Varghese, E.D. Jemmis, A. Venugopal Eur. J. Inorg. Chem. 2019 (2019) 3265-3269.
6. A theoretical analysis of the structure and properties of B26H30 isomers: Consequences to the laser and semiconductor doping capabilities of large borane clusters. Macháček, Jan, Antonio Francés-Monerris, Naiwrit Karmodak, Daniel Roca-Sanjuán, Jindřich Fanfrlík, Michael GS Londesborough, Drahommír Hnyk, and Eluvathingal D. Jemmis. Phys. Chem. Chem. Phys. 21 (2019) 12916-12923.
7. Overlap of Radial Dangling Orbitals Controls the Relative Stabilities of Polyhedral BnHn-x Isomers (n= 5-12, x = 0 to n – 1). N. Karmodak, R. Chaliha, E.D. Jemmis. Inorg. Chem. 58 (2019) 3627-3634.
